Hydrogen Cannon
 This experiment demonstrates the splitting of water into Hydrogen and Oxygen by electrolysis, and the re combining of these elements in an energetic reaction.
This experiment demonstrates the splitting of water into Hydrogen and Oxygen by electrolysis, and the re combining of these elements in an energetic reaction.
You can never recover the full energy from the Hydrogen fuel due to the lack of efficiency in the process used to make the hydrogen gas. During the electrolysis a current is flowing in the water to seperate the Hydrogen from the Oxygen, it is this current that produces wasted energy in the form of heat.
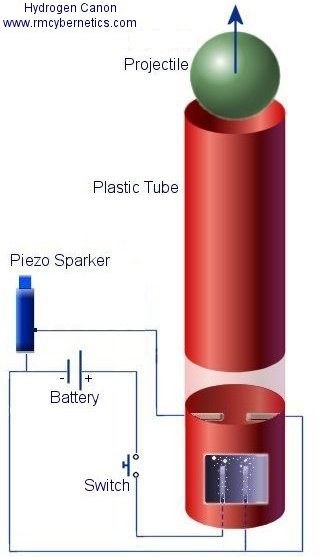
A plastic tube with one closed end has two electrodes inserted in the base so that they can be connected to wires on the outside. The electrodes should be made from carbon, but any conductor such as thick wire will do for this experiment. The space between the electrodes should be as small as possible but without them touching each other.
When the device is complete the tube should be filled with water until it just covers the electrodes. When the switch is pressed, the battery is connected to the electrodes and the flowing current begins to split the water into Hydrogen and Oxygen.
At some distance above the electrodes there are two more wires that are used for creating a spark to detonate the Hydrogen and Oxygen gas inside. The Piezo Sparker comes from a cheap lighter with click type ignition. When this is pressed it creates a high voltage that is used to create a spark. The two wires inside should be separated so that the spark will jump between them, and should be far enough from the water so that they don’t get drenched when the water is bubbling.
The projectile should fit snugly inside the end of the tube so that it doesn’t fall down to the water. It is important that this object is able to move freely enough to to leave the tube without a lot of resistance or else the whole thing could explode. The best type of projectile is a tube like the main one but slightly smaller so that it fits inside. If it is inserted so that the open end is down inside the larger tube the Hydrogen/Oxygen gas can build up behind it. As the tube is forced out when the gas is detonated it is forced to move in a straight line and can be more accurate than other shaped projectiles.
To fire the cannon the switch should be pressed for around 20 seconds depending on the electrodes and battery used. When enough gas has built up inside, the piezo sparker can be pressed detonating the H2 and O2 gas mixture. The gas expands rapidly forcing out the projectile and the product of the energetic reaction is just water again. The energy released in the explosion is almost the same as the energy supplied by the battery but it is released in a fraction of a second.






Years ago I had a lot of interest in electrolysis and came across a good reference studying the subject. As I recall the most efficient voltage was just under 1.5V at STP, and varies with temperature and pressure. To increase output increase the current. To reduce the resistance they used potassium hydroxide KOH. Why it was the preferred chemical I don’t recall. Also to reduce the resistance the plates should be placed very close together. If separating the gases a fine stainless steel mesh can be placed between the electrodes. As long as it stays submerged the bubbles are too large to pass thru. Stainless steel makes decent electrodes, but I think they used nickel in the commercial operations.
With any luck I could find the photo copies I made of parts of the book. With even better luck it might actual have a page that identifies the original. I was a teen when I found it and did not want to pay for any more copies than I absolutely needed…
what if i step down the voltage and d current increases?
Sad to see the hydrogen fueled car not being pushed for. Even though the initial source would have been powered via fossil fuels, it would have been a huge win on many levels. First, hydrogen burns cleanly and less emission controls would be needed on each vehicle to reach current tailpipe standards. Second, it is much easier to implement state-of-the-art pollution controls on the handful of power generation stations. Contrast that with million of engines. Third, it opens the door to extremely clean fuel cell based autos. Forth, at some point we will get our energy from something other than fossil fuels. Some of those methods may not produce energy 24/7. Storing energy produced by sources like solar as hydrogen is a handy way to hedge against a rainy day.
No, it would reduce current. More current = more gas
Could you use a voltage multiplier circuit to put more voltage into it to get more gas?
Cmon guys, They sell PWM’s and voltage boosters here and you have this 100 year old contraption and the Faraday laws cited. Just using a couple of devices from this site will greatly increase the efficiency and make this baby rock! And if you want to see that Stan’s Water Fuel Cell works, read his patents a few hundred times and build one. It’s not that hard plus it really works!
See this page about electrolysis
How can you calculate or measure the amount of hydrogen that is produced?
Yes, Hydrogen is combined with oxygen from the air as it passes through a fuel cell. During this process the fuel cell grabs electrons that are liberated during the reaction. The electrons are then used to drive an electric current.
The remains or exhaust are just a product of the reactants H and O, which of course is H2O (water)
If you need hydrogen and oxygen to create H2O and you burn the hydrogen in a fuel cell then all you would have left is oxygen. If all you have left is oxygen then how would the fuel cells exhuast be H2O. Just a question i have pondered for a while.
k thanx
Adding salt will make the solution more conductive and thefore it will make more H and O. It will also create other compounds from the salt. Possibly Chlorine gas and Sodium Hydroxide.
If you use copper electrodes, these will also become part of the reaction. They will get slowly eaten away or coated in something else. It will work for a while, but your solution will eventually turn to sludge.
if i were to add salt to the mixture would it still make hydrogen and oxygen or wat?also if i don’t use salt in the water,will i need the carbon sticks or watever,out of a battery, or can i just use copper wire?
Almost any voltage will work. The higher the voltage, the faster the gas will be produced.
i was wondering wat voltage of battery would be best to use in this experiment?
The problem is that a battery does not hold enough energy compared to petrol or other fuels.
Several people have claimed to make cars that run on water. One famous case is of Stanley Meyer who was sued by his investors when his car could not be proved to work. There was infact a program about it titled “It Runs on Water”
The plans for future hydrogen cars are supposedly going to reduce pollution as the exhausts will only be water. This would be true if the hydrogen was going to be made by splitting water with wind or solar power. Unfortunatley President Bush actually intends to power the electroysis plants with fossil fuels! This means that the whole hydrogen car thing is just a way to mislead people into thinking that Bush wants to reduce fossil fuel consumption. See here
Why couldn’t this simple small version be applied for use in car engines where a piston would replace the projectile? Seems fairly simple and the water (maybe salt water) would act as the catalyst. A larger battery would be required and cold be used with a capacitor such as a photo flash type. With the proper finances and parts, a auto company could easily make a car that could be titled “Runs on water!” ~ MasterMindUser@MSN.com